Description
Ensuring the health and longevity of catheters is a paramount concern, and a dependable taurolock catheter lock solution plays a crucial role. TauroLock HEP500 Vial steps in as an advanced solution, elevating catheter care to new heights. With the continuous advancement in medical technology, the demand for efficient catheter maintenance has surged, making innovative solutions like TauroLock HEP500 Vial indispensable. Discover the convenience of purchasing TauroLock HEP500 Vial online in Pakistan.
TauroLock HEP500 Vial is specifically designed to maintain catheter patency and reduce the risk of catheter-related infections. Its formulation acts as a protective barrier, preventing clot formation and microbial colonization, thus extending catheter lifespan. Whether you’re a healthcare professional aiming for the best patient care or an individual managing a catheter at home, TauroLock HEP500 Vial ensures optimal catheter maintenance.
The escalating demand for quality healthcare products has led to the significance of online accessibility. Now, acquiring TauroLock HEP500 Vial online in Pakistan is a simple process. With just a few clicks, you can contribute to the well-being of catheter-dependent individuals and enhance their quality of life.
At Algene Medical, our commitment to delivering impactful healthcare solutions remains unwavering. TauroLock HEP500 Vial stands as a testament to our dedication to innovation and excellence in medical care. With patients and healthcare providers at the forefront of our minds, we proudly present TauroLock HEP500 Vial as the ultimate catheter lock solution.
In summation, TauroLock HEP500 Vial epitomizes catheter care innovation. Its ability to prevent clot formation and microbial colonization makes it an indispensable component of catheter maintenance. Embrace the convenience of purchasing TauroLock HEP500 Vial online in Pakistan and join us in promoting enhanced healthcare for catheter-dependent individuals. With TauroLock HEP500 Vial, your catheter care journey is elevated to the highest standards.
With 500 IU/ml of heparin, TauroLock™-HEP500 is particularly suitable for those who want to minimise occlusion rates throughout dialysis treatment.
TauroLock™-HEP500 is to be used with a catheter-based vascular access device for hemodialysis.
This type of lock solution prevents the formation of bacterial biofilms.
Therefore, patients remain protected from catheter-related infections and ensure a high patency rate at the same time.
Contraindications
In some cases, we do not recommend using TauroLock™-HEP500 for vascular access systems (VAD):
– The patient has been diagnosed with HIT.
– The patient has displayed a hypersensitive reaction to citrate or taurolidine.
– The patient is taking medication which interferes with either citrate, heparin, or taurolidine.
Alternatively, you may rely on TauroLock™ and/or TauroLock™-U25.000 to prevent catheter-associated complications.
Safety
Prior to the next treatment, TauroLock™-HEP500 must be aspirated.
Package Sizes
If you would like to purchase TauroLock™-HEP500, there are two options available:
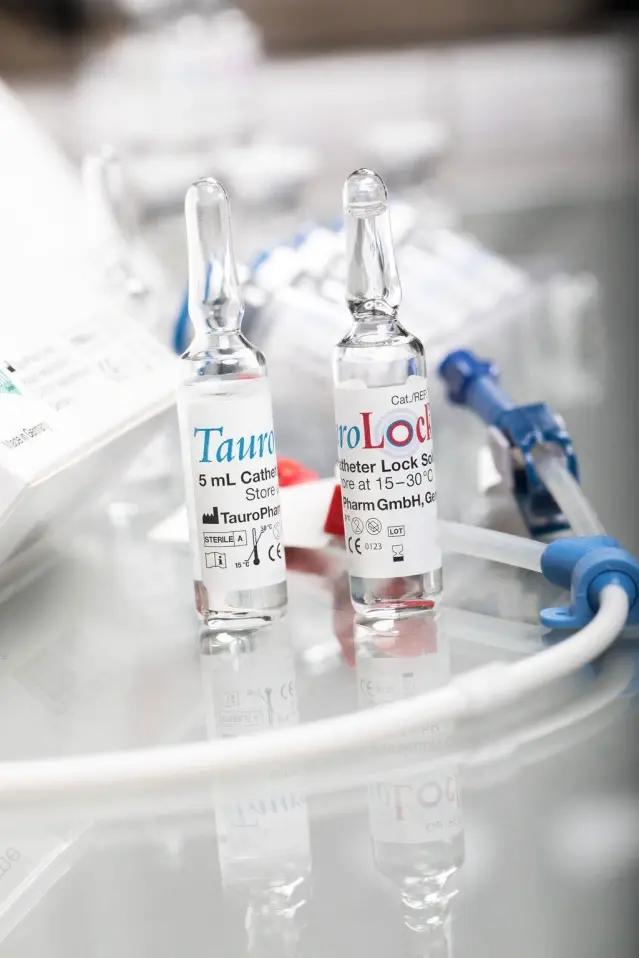
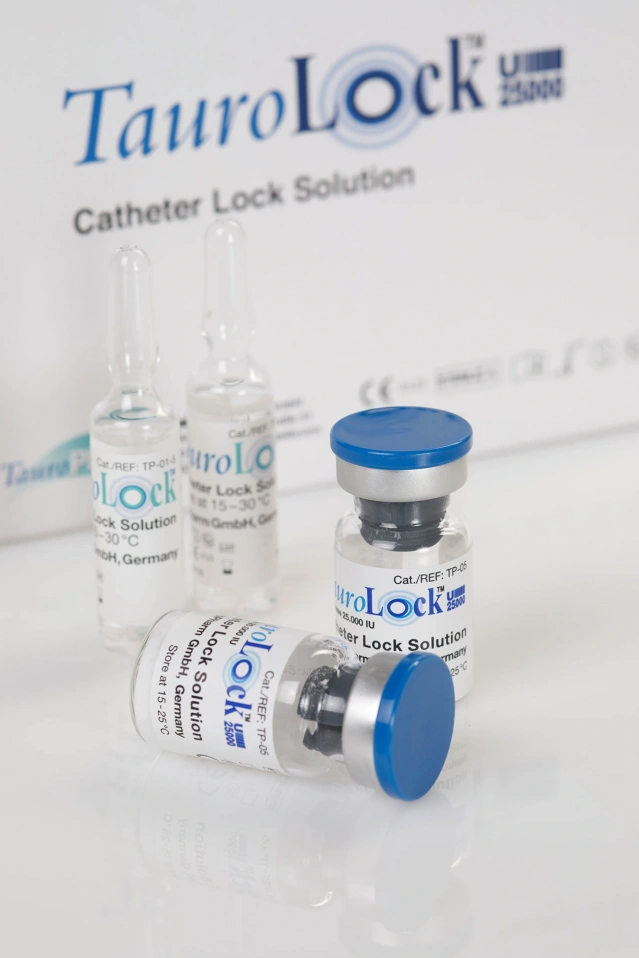
– ampoules of 5 ml (single dose, 10 ampoules per box)
– vials of 10 ml (mulitple dose, 100 vials per box)
Storage & Transport
TauroLock™-HEP500 must be stored at 15 °C to 30 °C.
Please note that you must not freeze TauroLock™-HEP500 under any circumstances.

Jasmine Block, Bahria Town
Lahore 53720, Pakistan
Faq's
NutriLock™ and TauroLock™ are catheter lock solutions for tunneled and non-tunneled vascular access and port systems for the prevention of catheter-associated infections and catheter flow problems.
NutriLock™ contains taurolidine as an antimicrobial ingredient. In addition to taurolidine, TauroLock™ solutions also contain 4 % citrate for the maintenance of catheter patency.
In addition to taurolidine and 4 % citrate, TauroLock™-HEP100 contains additional 100 IU/ml heparin to improve patency. TauroLock™-HEP500 contains additional 500 IU/mL heparin.
Тhe most effective prophylaxis against catheter occlusion is maintained with the regular use of TauroLock™-U25.000, containing 25.000 IU urokinase (5.000 IU/mL), taurolidine and 4 % citrate. This reduces flow problems in the catheter substantially (see lock solutions in dialysis).
The decision as to which catheter lock solution is most advantageous depends on the patient’s individual situation. Alternating use (e.g. TauroLock™-HEP500, TauroLock™-U25.000) is feasible.
Heparin and 4 % citrate only have anticoagulant effects, but no bactericidal properties. Consequently, contamination of the catheter can lead to bacteraemia.
TauroLock™ solutions are used to prevent both infections and occlusions in catheter and port systems. NutriLock™ acts prophylactically against catheter infections.
Prophylactic use of TauroLock™ solutions prevents the formation of biofilm and thus maintains the flow rate in the catheter and/or port. The fibrinolytic activity of TauroLock™-U25.000 further improves patency by dissolving already formed clots.
Due to the citrate content (citrate content: 4 %), overly rapid application into the bloodstream can lead to mild hypocalcaemic effects (e.g. metallic taste).
A citrate concentration of 4 % is recommended by US regulatory authorities and others.
- In the context of one case of fatality, the FDA issued an advisory not to use a product with a higher citrate content (Tricitrasol, 46.7 %). Lock solutions of this type had to be recalled from the US market. Their use is no longer approved.
- Likewise, over-instillation of less than 1 ml per lumen of a 30% citrate solution induced transient cardiac arrest in two cases in the Netherlands (Punt CD, Boer WE, Cardiac arrest following injection of concentrated trisodium citrate, Clinical Nephrology 2008, 69 (4), 317-318).
- A high citrate content (46.7%) can also lead to embolic events which might be triggered by protein precipitation (Davenport A , Willicombe MK, Vernon K, Embolic complications from central venous hemodialysis catheters used with hypertonic citrate solution, American Journal of Kidney Diseases, 2010, 55, 348-351 / Schilcher G, Scharnagl H, Horina JH, Ribitsch W, Rosenkranz AR, Stojakovic T and Polaschegg H-D, Trisodium citrate induced protein precipitation in haemodialysis catheters might cause pulmonary embolism, Nephrol Dial Transplant (2012) 0: 1–5).
Instructions and guidelines
- Before instilling the lock solution, flush the vascular access system with at least 10 ml of physiological saline with the pulsatile flushing technique.
- Use a suitable syringe to remove NutriLock™ or a TauroLock™ solution from the container.
- Instill the lock solution into the access device in a quantity sufficient to fill the lumen completely. The specific filling volume is stated in the instructions for use of the access system.
- NutriLock™ or TauroLock™ solutions remain inside the access system until the next treatment.
- Before starting the next treatment, the lock solution must be aspirated.
- Flush the vascular access system with 10 ml of physiological saline.



Reviews
There are no reviews yet.